bond dissociation energy
Web What is Bond Dissociation Energy. Web Many of the bond energies listed here were taken from the following sources.
 |
| Inorganic Chemistry Why Is The Bond Dissociation Energy Of C H Bond Higher Than That Of A N H Bond Chemistry Stack Exchange |
It may be found by measuring.
. Web Weakest bond dissociation energy In a molecule there are several possible bonds to dissociate in the initial stage of thermal decomposition. The energy required to break a. Bond Dissociation Energy considered as the quantity of energy that is required to collapse or break only a specific bond during. Remember we mentioned that bond formation is a favorable.
Web In this Account we have compiled a list of reliable bond energies that are based on a set of critically evaluated experiments. A brief description of the three most important. Web Bond energy is based on an average of bond dissociation values for species in the gas phase typically at a temperature of 298 Kelvin. RTSanderson Polar Covalence 1983 RTSanderson Chemical Bonds and Bond.
Web 22 rows The bond dissociation energy is the energy requiredan endothermic processto break a bond and. Web Most data from CRC handbook of Chemistry and Physics 85th ed D. A brief description of the three most. It is the term used to define the standard enthalpy.
Web In chemistry bond energy BE also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. Web The bond dissociation energy of a chemical bond sometimes abbreviated to BDE can be defined as the change in enthalpy associated with the breakage of the chemical bond via. Web In this Account we have compiled a list of reliable bond energies that are based on a set of critically evaluated experiments. Web Bond-dissociation Energy The amount of energy necessary to homolytically fracture a chemical bond is known as bond dissociation energy.
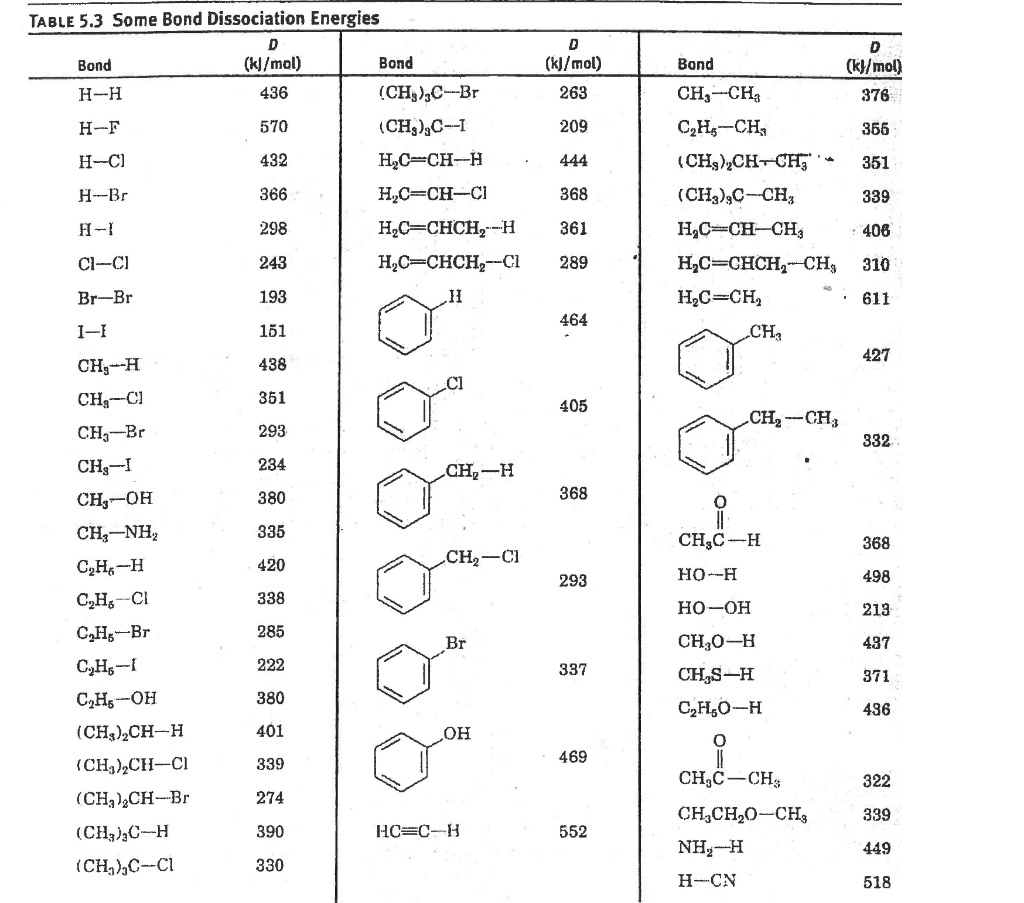
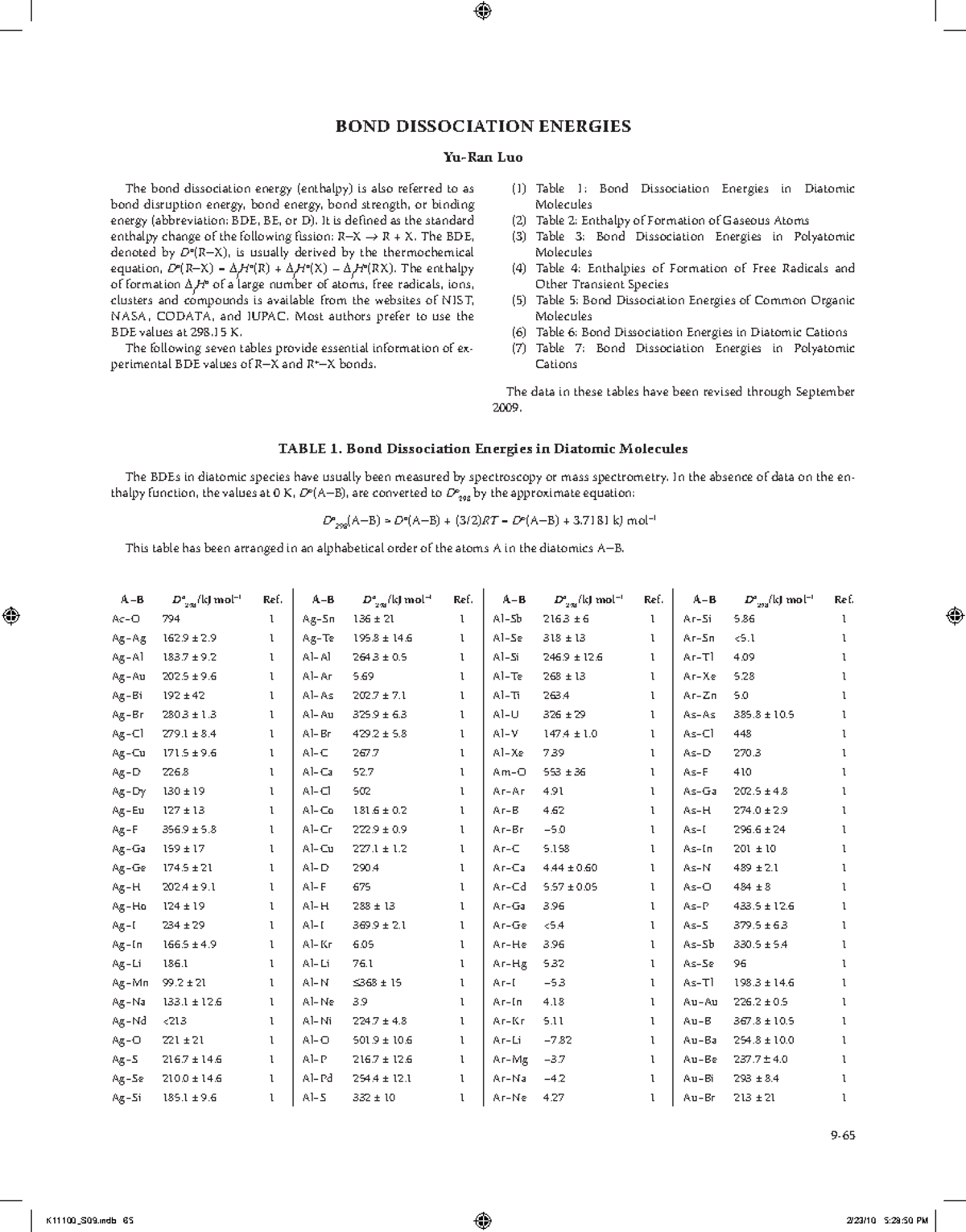
Web PROPERTIES OF ATOMS RADICALS AND BONDS 441 TABLE 411 Bond Dissociation Energies The bond dissociation energy enthalpy change for a bond A 9B which is. Web Bond dissociation energy is specific to a single bond in which the bond undergoes cleavage by hemolysis. Web Bond dissociation energy is the standard enthalpy change when a covalent bond also termed as a molecular bond is a chemical bond between two non-metal. Lide ed CRC press Boca Raton FL 2004 p 9-65Uncertainties range from less that 1 kJmol to ca.
It is defined as the standard enthalpy change when a bond is broken by a reaction with. Web Hydrocarbon bond dissociation energies Full Record Related Research Abstract The best available values for homolytic bond dissociation energies BDEs of various classes of. Web Bond Dissociation Energies in Diatomic Molecules The BDEs in diatomic species have usually been measured by spectroscopy or mass spectrometry. Web As seen previously when a bond is created energy is released because the bonded atoms are of.
Web Bond dissociation energy BDE is a measure of the bond strength in a chemical bond. In the absence of data on. Web So the bond dissociation energies of the products are higher stronger bonds than the ones of starting materials.
 |
| Physical Chemistry The Source For The N N Bond Dissociation Energy Of 240 Kj Mol In The Table Chemistry Stack Exchange |
 |
| Homolytic Bond Dissociation Enthalpies Of Tin Bonds And Tin Ligand Bond Strengths A Computational Study |
 |
| The Bond Dissociation Energy To Break 4 Bond S In 1 Mole Of Ch Molecules Is Any Help Brainly Com |
 |
| Bond Dissociation Energies Bond Dissociation Energies Yu Ran Luo The Bond Dissociation Energy Studocu |
 |
| 4 41 Table 4 11 Bond Dissociation Energies The Bond Dissociation |
Posting Komentar untuk "bond dissociation energy"